
Quality
Quality Control for Pharmaceutical Packaging Production
Aphena’s commitment to compliance with both current Good Manufacturing Practice (cGMP) and customer requirements ensures a proactive approach on every project and product. The leadership systems provide a mechanism for organizational learning and process control. Quality team members are knowledgeable and trusted high performers.
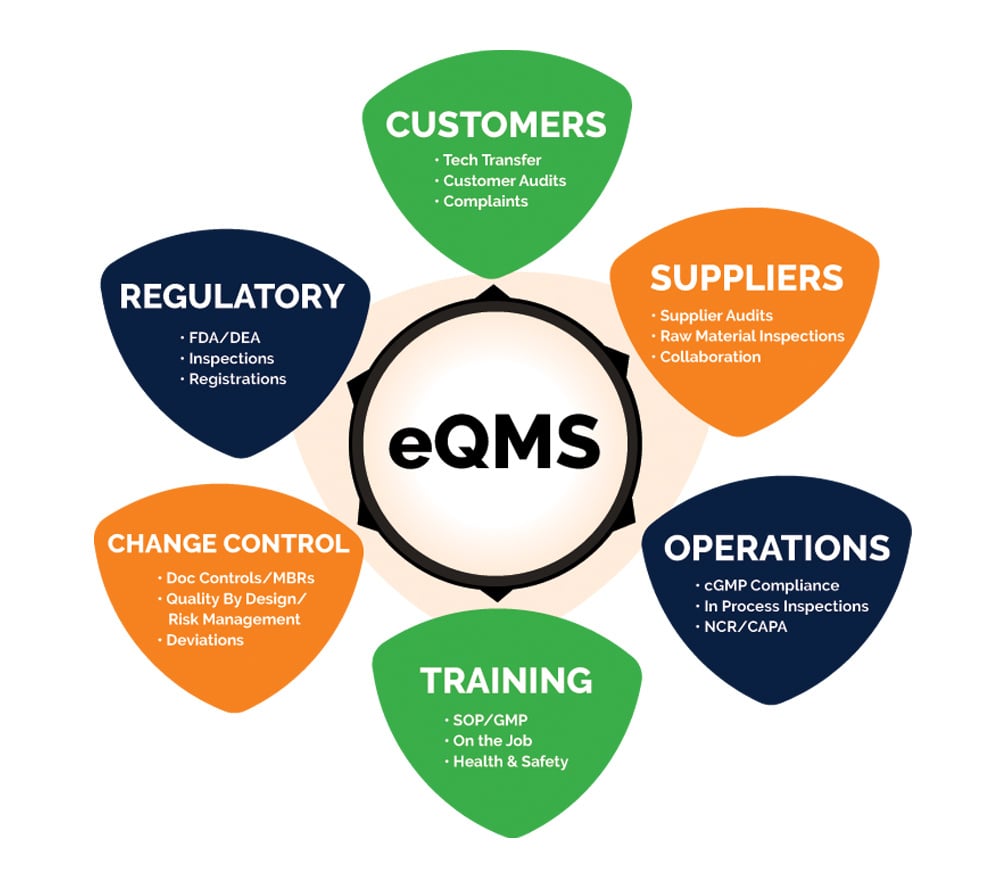
All operations are supervised by full-time quality control specialists and undergo rigorous validation and safety checks. All production runs are in compliance with cGMP and other FDA regulations. A new electronic Quality Management System (eQMS) supports all quality assurance processes, procedures and responsibilities for Aphena’s operation and customers.
At Aphena, the quality assurance leadership and operational personnel report directly to the CEO. The data-driven systems and improvement strengthens the Aphena quality team year over year. When issues arise, the same data-driven techniques identify and correct root causes in the event of any nonconformance issue.

Aphena
Analytical Lab
Aphena’s Liquids & Topicals Division utilizes on-site analytical & micro laboratories as part of our full-turnkey manufacturing & packaging services of liquids, creams, gels, suspensions, foams and lotions.

Aphena
Credentials
Aphena's personnel, equipment and facilities are guided by exacting processes and procedures. Aphena's facilities are all FDA registered.

Aphena
Quality Systems
In addition to our outstanding credentials, Aphena has many systems in place to ensure quality during the process and production of every product.
