
Credentials
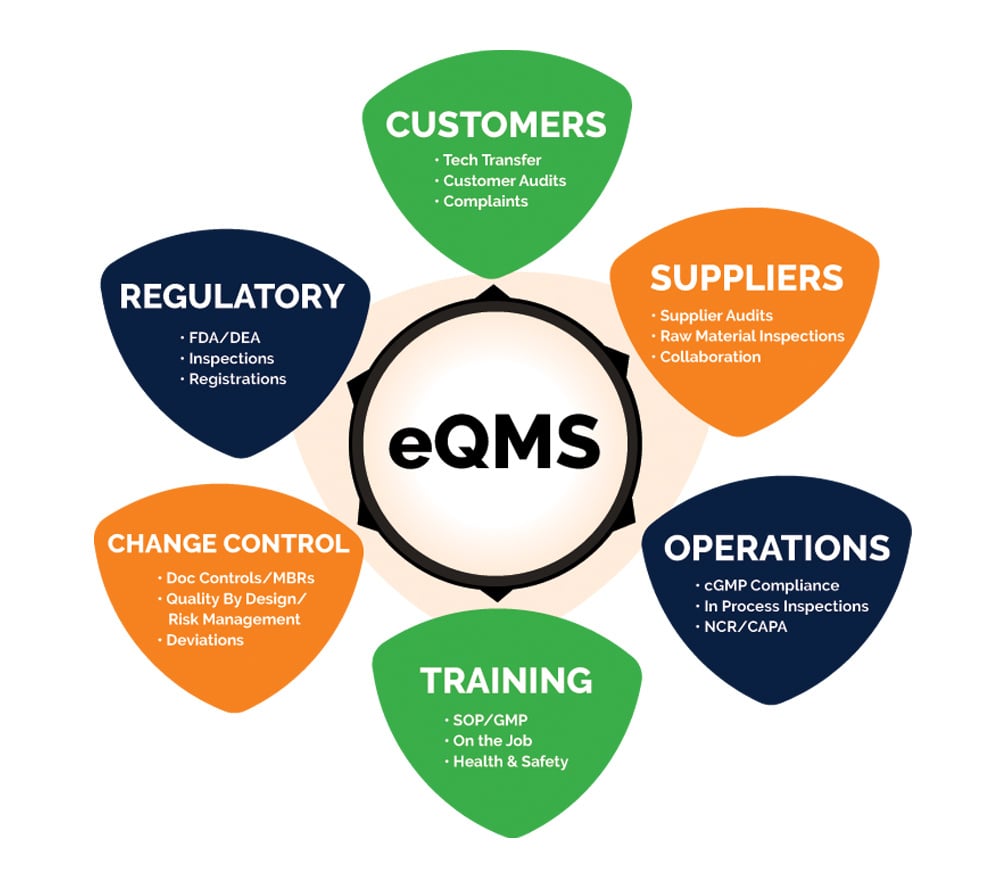
Aphena Pharma Solutions’ personnel, equipment and facilities are guided by exacting processes and procedures to provide our clients with the best in contract pharma packaging and manufacturing services. Aphena's facilities are all FDA registered. The Tennessee site is DEA and State Board of Pharmacy licensed. The FDA's current Good Manufacturing Practice (cGMP) governs standard operating procedures. The organization meets and exceeds FDA standards for 21 CFR, Parts 111, 210, 211 and 820 and DEA-licensed locations (CII-CV).
- FDA Registered / FDA Inspected / cGMP Compliant
- DEA-Licensed Locations (CII-V)
- Handling NDA, ANDA & OTC Drugs
- Board of Pharmacy Licenses
- ISO 13485:2016 & ISO 9001:2015 (BSI) Registered
- eQMS System
- DSCSA Compliant
- Serializing Hardware - Optel
- Serialization Software RfXcel
- UL Audited & Approved
- Approved by all Major Retailers
- LIMS System
